
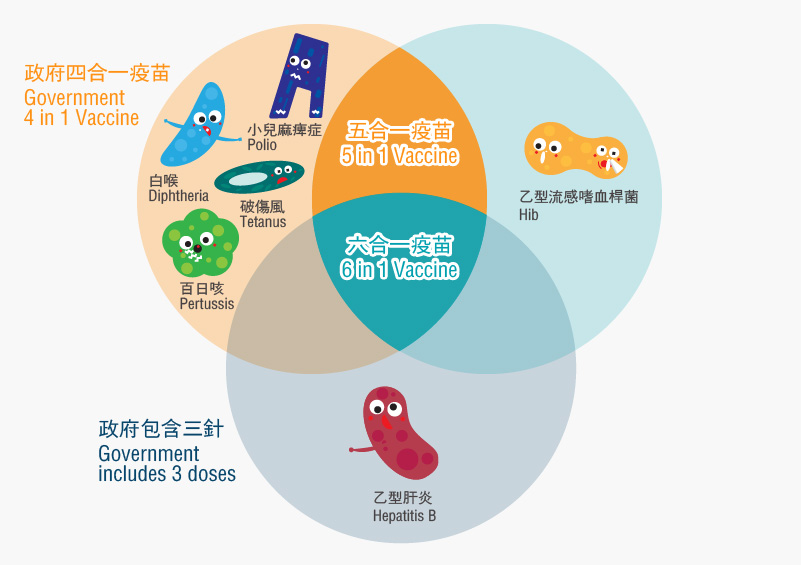
The 6-in-1 vaccine is a combination of vaccines that can prevent 6 diseases.
The six diseases were diphtheria, tetanus, pertussis (whooping cough), polio, Haemophilus influenzae type B and hepatitis B.
Age |
Government Vaccination Program |
Recommended vaccination schedule |
| Newborn | Hepatitis B Vaccine (1st dose) - Injected at birth | |
| 1st Month | Hepatitis B Vaccine (2nd Dose) |
-
|
| 2nd Month |
4-in-1 vaccine (1st dose) |
*Injected at 6-8 weeks* 6-in-1 vaccine (1st dose) |
| 4th Month |
4-in-1 vaccine (2nd dose)
Haemophilus influenzae type B vaccine (2nd dose) *injection in private hospital
|
6-in-1 vaccine (2nd dose) |
| 6th Month |
4-in-1 vaccine (3rd dose)
Haemophilus influenzae type B vaccine (3rd dose) *injection in private hospital
Hepatitis B Vaccine (3rd Dose) |
6-in-1 vaccine (3rd dose) |
| 18th Month |
4-in-1 vaccine (4th dose)
Haemophilus influenzae type B vaccine (3rd dose) *injection in private hospital
|
5-in-1 vaccine (booster dose) |
|
|
Total 11 injections | Total 5 injections |
The 6-in-1 vaccine combines Haemophilus influenzae type B and hepatitis B injections. In addition to preventing diphtheria, tetanus, pertussis, and polio, it can also prevent Haemophilus influenzae type B and hepatitis B, reduce the total number of injections by up to six , but also minimize the chance of side effects and discomfort.
Vaccine name |
Infanrix-hexaTM |
Infanrix-IPV-HIBTM |
Pharmaceutical company |
UK GSK | |
Suitable for |
Haemophilus influenzae type b infection needs to be prevented People who want to reduce the number of injections |
|
Effective Prevention |
Diphtheria, Tetanus, Pertussis, Polio, Haemophilus influenzae type B and Hepatitis B | |
Vaccination period |
It is recommended that babies start their first injection of the 6-in-1 vaccine when they are 6-8 weeks old After that, the second and third injections were given at 4 and 6 months respectively when the baby is 18 months old, a 5-in-1 vaccine booster injection will be given |
|
Side effect |
Loss of appetite, irritability, pain at the needle site, redness and swelling (less than 50 mm), mild fever and fatigue may occur after vaccination, but they will recover quickly | |
Not Suitable for |
Hypersensitivity to any component of the vaccine If encephalopathy of unknown etiology occurs within seven days of pertussis vaccination, pertussis vaccine should be discontinued immediately, but vaccination for other diseases should continue |
|
Precautions |
If the following situations occur after getting vaccinated with pertussis vaccine, medical staff should carefully consider whether to give pertussis-containing vaccine Fever over 40°C within 48 hours of vaccination, not caused by other reasons Symptoms of syncope or shock occur within 48 hours of inoculation Prolonged, persistent crying (more than three hours) within 48 hours of vaccination Having seizure within 3 days of vaccination (with or without fever)
**When Infanrix-hexaTM / Infanrix-IPV-HIBTM is in short supply, the company has the opportunity to replace it with a vaccine of the same quality - Hexaxim/Pentaxim (Sanofi, France). |
|

|
|

完成測試後,有機會獲免費骨質密度檢查 (DEXA) 乙次 或 骨質密度檢查優惠券乙張
閣下資料將會用作此推廣活動聯絡用途,如因資料有誤而未能聯絡閣下,本公司一概不負上任何責任。